 |
|
|
|
| |
|
|
| |
|
Nickel sulfide is the inorganic compound with the formula NiS.
It is a black solid that is produced by treating nickel (II) salts with hydrogen sulfide .
Many nickel sulfides are known, including the mineral millerite .
Nickel sulfides are related to this and other minerals, are the products of desulfurization reactions, and are sometimes used as catalysts .
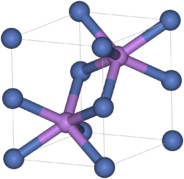
Like many related materials, nickel sulfide adopts the nickel arsenide motif.
In this structure, nickel is octahedral and the sulfides are in trigonal prismatic sites.
Nickel sulfide has two polymorphs .
The alpha form has a hexagonal unit cell, while the beta form has a rhombohedral cell.
Small amounts of this compound occur as imperfection when glass is manufactured.
It is believed that cracks occur in toughened window glass panels occur due to the volume change associated with the alpha to beta phase transition of these nickel sulfide defects.
|
■ General Properties
○ Molecular Formula : NiS
○ Molar mass : 90.7584 g mol-1
○ Appearance : black solid
○ Odor : Odorless
○ Density : 5.8 g/cm3
○ Melting point : 797 °C
○ Boiling point : 1388 °C
○ Solubility in water : nsoluble
|
|
|
|
|

Working days : Monday to Saturday

|
|
|
